
An inertelectrode’s ability to electrolysis depend on the reactants in the electrolyte solution while an activeelectrode can run on its own to perform the oxidation or reduction half reaction.The standard condition is to have a pH of 4 in the anode half cell but sometimes during nonstandard states, the pH may be higher or lower changing the voltage.This means that the reduction of chloride = 1.31V not 1.36V A high-temperature, secondary electrochemical cell includes a negative electrode containing an alkali metal such as lithium, an electrolyte of molten salt.

Concentration of chloride ion = 5.5M not the unit activity of 1M.The reactants may be in nonstandard conditions which means that the voltage for the half cells may be less or more than the standard condition amount.There might be more than one electrode reaction that occurs meaning that there may be more than one half-reaction leaving two or more possibilities for the cell reaction.H 2 (g) requires a 1.5 V overpotential, while Pt (s) requires 0 V overpotential
This case happens more frequently with gases.
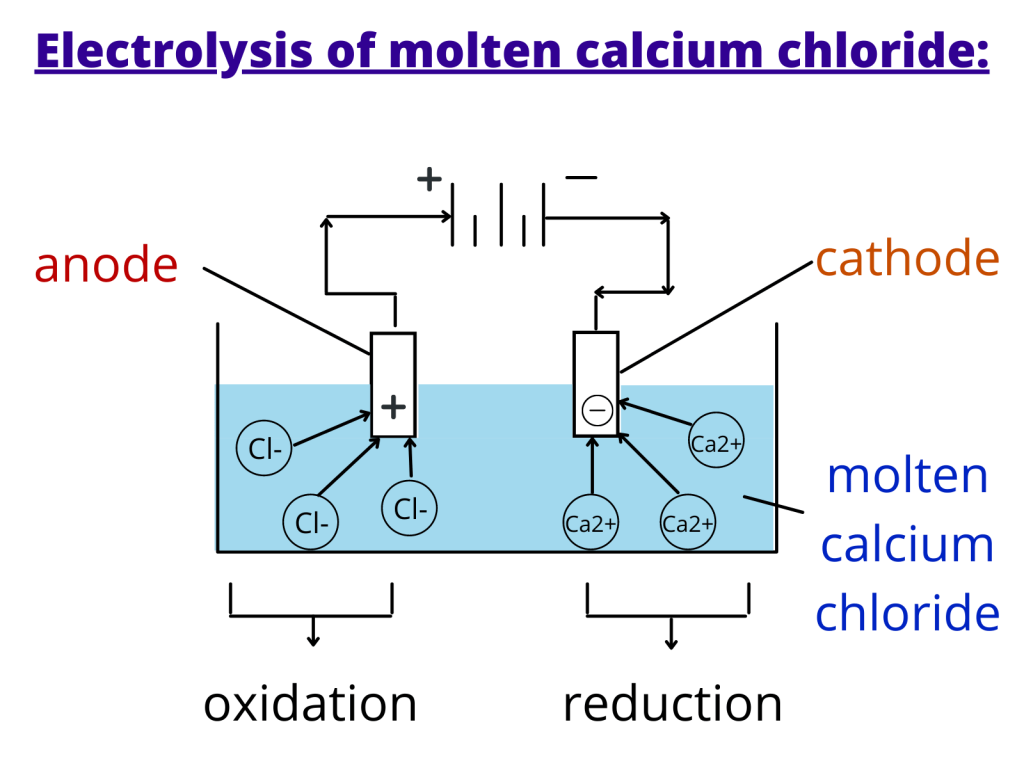
An overpotential or voltage excess is sometimes needed to overcome interactions at the electrode surface. There are four primary factors that determine whether or not electrolysis will take place even if the external voltage exceeds the calculated amount: If an aqueous solution of sodium chloride were used in the above system, hydrogen would undergo reduction instead of sodium, because it is a stronger oxidizing agent that sodium. The substance that is the strongest oxidizing agent will be reduced. The substance that is the strongest reducing agent (the substance with the highest standard cell potential value in the table) will undergo oxidation. True False Electrolysis of molten KCl, At anode, 2ClCl2+2e at cathode. The conditions under which the electrolyte cell operates are very important. Anode is now positive charged and the cathode has a negative charged. Note that the site of oxidation is still the anode and the site of reduction is still the cathode, but the charge on these two electrodes are reversed. Our results demonstrate that such dual-phase composites PBC–GDC produced in one step can be considered a promising cathode material for intermediate/low-temperature solid oxide fuel cells.+ 2e^-\] The maximum power density of lab-scale electrolyte-supported cell with PBC–10 wt% GDC cathode reaches 1302, 938, 549 and 235 mW cm −2 at 850, 750, 650 and 550 ☌, respectively. The GDC introduction significantly decreases the charge transfer resistance of the PBC–GDC composite cathode. Many studies have focused on the anode performance, while little attention was paid to the cathode. The charge transfer process on the electrode surface is the rate-limiting step based on the dependence of polarization resistance at different oxygen partial pressures, where the impedance analysis technique, the distribution of relaxation times (DRT), is devoted to describing the complex electrode reaction processes. Abstract Molten carbonate direct carbon fuel cells (MC-DCFCs) are among the most promising devices for high-efficiency energy conversion and clean power generation from coal. When measured in a symmetrical cell configuration in the air at 600 ☌, PBC–10 wt% GDC electrode shows an area-specific resistance of 0.394 Ω cm 2, which is about 78% lower than that of bare PBC electrode under the same situations. The introduction of the cubic GDC phase can guide the oxygen transport among PBC particles with different orientations. The coherent interface structure is formed between PBC and GDC particles, which is beneficial to alleviate the lattice thermal expansion. develop suitable cathode materials to accelerate the cathode reaction rate and to improve the cell performance.1,2 As shown in eqn (1) and (2), cathode reactions for O-SOFCs and P-SOFCs are quite dierent and thus have dierent requirements on their cathode materials. Here, we report a dual-phase cathode material, double perovskite structure PrBaCo 2O 5+ δ (PBC) and fluorite structure Gd 0.1Ce 0.9O 2− δ (GDC), successfully synthesized using a one-pot method, with remarkable oxygen reduction reaction activity and low polarization resistance under IT-SOFC service conditions. One challenge facing the development of high-performance cathodes for solid oxide fuel cells is the slow oxygen reduction kinetics.






 0 kommentar(er)
0 kommentar(er)
